Refrigerators
A refrigerator is a cyclic machine, corresponding to the reverse of an engine: Work is performed to remove heat qc from a system at the temperature Tc (the inside of the refrigerator), making it colder, and transfer it the heat qh to the surrounding at Th.
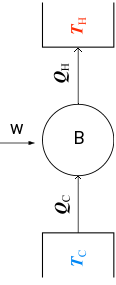
In a cycle ΔU = 0, thus because the system absorbs w and qc, the energetic balance is
|w| + |qc| = |qh|
The coefficient of performance K of a refrigerator expresses the performance of the refrigerator, defined as
K = qc / w
The designer of a refrigerator aims to extract as much heat qc as possible.
Any substance used in a refrigeration machine to produce cooling by evaporation is called a refrigerant (as freon). The refrigeration cycle includes the alternate evaporation and condensation of a refrigerant. A pressure differential between two regions separated by a valve is generated by a compressor. When the refrigerant flows through the expansion valve, the liquid refrigerant is allowed to move from a high-pressure zone to a low-pressure. With the pressure reduced, the refrigerant evaporates and absorbs heat from the interior of the system. The compressor circulates the vaporized refrigerant to the exterior condenser coils, where it is condensed by pressure. Heat is released during condensation to the surroundings.
Energy is transferred as heat qc to the working substance from the low-temperature reservoir (inside of the refrigerator) and is transfered as heat Qh to the high-temperature reservoir (the surroundings). Thus it is necessary the condenser to be located in the surroundings, and the evaporator inside the system (Fig.2).
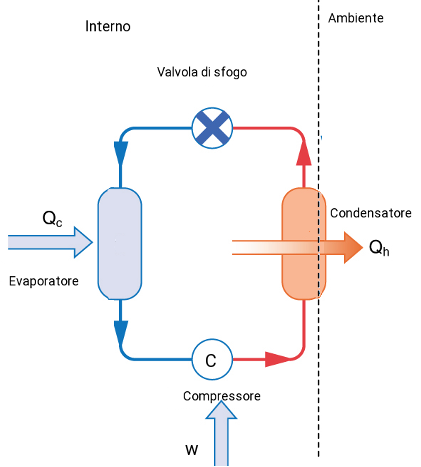 Fig. 2 Functioning of a refrigerator.
Fig. 2 Functioning of a refrigerator. The condenser is located in the environment
The choiche of the refrigerant is based on the capacity of the fluid to evaporate at low-temperatures and pressures. Another common refrigerator is 1,1,1,2-tetrafluoroetano, whihc boils at T = −26.1 °C. In the past refrigerants containing chlorine were employed, which deplet the ozone in the atmosphere
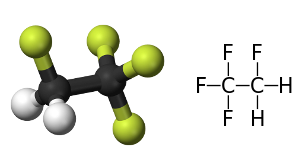 Fig. 1 1,1,1,2-Tetrafluoroethane a modern refrigerant.
Fig. 1 1,1,1,2-Tetrafluoroethane a modern refrigerant.can sustain repeated cycles of evaporation/condensation.
An heat pump is a refrigerator working in reverse, which is normally used to heat up an environment. In a heat pump the condener is placed in the surroundings, whilst the evaporetor in the system., thus Qh, is absorbed inside the system Fig. 3.
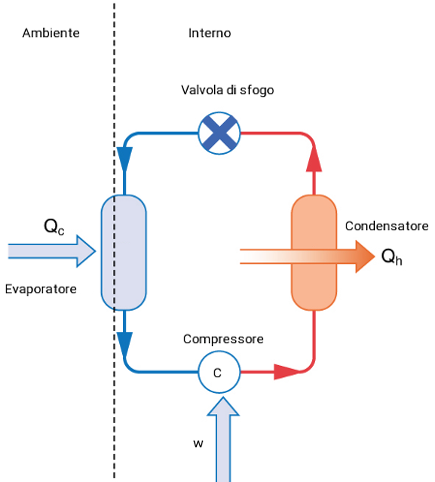 Fig. 3 Functioning of an heat pump.
Fig. 3 Functioning of an heat pump. L'evaporatore è a contatto con l'ambiente
Clausius statement of the second Law
It would be nice to own a refrigerator that did not require some input of work - that is, one that would run without being plugged in.
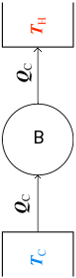
Figure 4 represents another "inventor's dream," a perfect refrigerator that transfers energy as heat q from a cold reservoir to a warm reservoir without the need for work. Because the unit operates in cycles, the entropy of the working substance does not change during a complete cycle. The entropies of the two reservoirs, however, do change: The entropy change for the cold reservoir is −|q|/Tc, and that for the warm reservoir is |q|/Th. Thus, the net entropy change for the entire system is
ΔS = −|q|/Tc + |q|/Th
Because Th > Tc, the right side of this equation is negative and thus the net change in entropy per cycle for the isolated system refrigerator + surroundings is also negatiefv. Because such a procees is forbidden by the second law of thermodynamic we can conclude that
It is impossible for a system to undergo a cyclic process whose sole effects are the flow of heat into the system from a cold reservoir and the flow of an equal amount of heat out of the system into a hot reservoir
.
which is known as the Clausius statement of the second Law. In short, there are no perfect refrigerators.
Proof of the equivalence of the Clausius and Kelvin–Planck statements is given next.