Measurement of Heat of Reaction
You measure the heat of reaction in a calorimeter, a device used to measure the heat absorbed or evolved during a physical or chemical change. The device can be as simple as the apparatus sketched in Figure 6.12, which consists of an insulated container (for example, a pair of polystyrene coffee cups) with a thermometer. More elaborate calorimeters are employed when precise measurements are needed for research, although the basic idea remains the same—to measure temperature changes under controlled circumstances and relate these temperature changes to heat.
The coffee-cup calorimeter shown in Figure 6.12 is a constant-pressure calorimeter. The heat of the reaction is calculated from the temperature change caused by the reaction, and since this is a constant-pressure process, the heat can be directly related to the enthalpy change, ΔH. Research versions of a constant-pressure calorimeter are available, and these are used when gases are not involved.
For reactions involving gases, a bomb calorimeter is generally used.
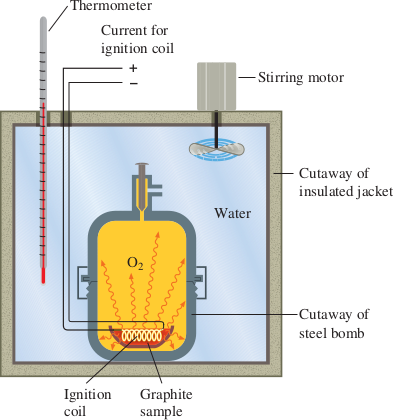
Consider the heat of combustion of graphite, the form of carbon used in the “lead” of a pencil. To measure the heat released when graphite burns in oxygen, a sample of graphite is placed in a small cup in the calorimeter. The graphite is surrounded by oxygen, and the graphite and oxygen are sealed in a steel vessel, or bomb. An electrical circuit is activated to start the burning of the graphite. The bomb is surrounded by water in an insulated container, and the heat of reaction is calculated from the temperature change of the calorimeter caused by the reaction.
Because the reaction in a bomb calorimeter occurs in a closed vessel, the pressure does not generally remain constant. Rather the volume remains constant, and under these conditions the heat of reaction does not in general equal ΔH; a small correction is usually needed. However, this correction is negligible when the reaction does not involve gases or when the number of moles of reactant gas equals the number of moles of product gas, as in the combustion of graphite to carbon dioxide.
Example 1. Calculating ΔH from Calorimetric Data Suppose 0.562 g of graphite is placed in a calorimeter with an excess of oxygen at 25.00°C and 1 atm pressure (Figure 6.13). Excess O2 ensures that all carbon burns to form CO2. The graphite is ignited, and it burns according to the equation
C(graphite) + O2 (g) ⟶ CO2 (g)
On reaction, the calorimeter temperature rises from 25.00 °C to 25.89 °C. The heat capacity of the calorimeter and its contents was determined in a separate experiment to be 20.7 kJ/°C. What is the heat of reaction at 25.00 °C and 1 atm pressure? Express the answer as a thermochemical equation.
Solution. The quantity of heat absorbed by the calorimeter is Ccal ΔT. This will have the same magnitude as qrxn, but the opposite sign
qrxn = −Ccal ΔT = −20.7 kJ/°C x (25.89 − 25.00 °C) = −20.7kJ/°C x 0.89 °C = −18.4 kJ
The heat per mole released by the reaction will be
12 g / 1 mol = 0.562 g/ x mol
x = 0.047 mol C
and
−18.4 kJ / 0.047 mol C = x kJ / 1 mol C
x = −3.9 x 102 kJ
We can summaize the result by the termochemical equation
C(graphite) + O2 (g) ⟶ CO2 (g); ΔH = −3.9 x 102 kJ